Neurotransmission at excitatory synapses involves the release of glutamate from vesicles, diffusion and binding of glutamate to receptors, and eventual uptake of glutamate by transporters (see schematic diagram below).
Transporter-mediated uptake is critical for terminating the actions of glutamate, preventing the sustained activation of receptors that would otherwise disrupt signaling at synapses and lead to excitotoxic neurodegeneration. At synapses in the CNS, glutamate transporters can influence the occupancy of glutamate receptors by reducing the peak concentration of glutamate within the synaptic cleft, by accelerating the decay of the glutamate transient, and by restricting the diffusion of glutamate to perisynaptic receptors or receptors at adjacent synapses. The ability of transporters to alter synaptic responses has been shown to depend on the structure of the synapse, the properties of the glutamate receptors, and the frequency of release. For synapses that contain a high density of release sites, such as calyceal synapses, or are tightly ensheathed by glial processes, transporter inhibition prolongs the decay of EPSCs. At synapses characterized by partial ensheathment by astrocytes and single release sites, transporter blockers have little effect on the decay of either AMPA or  NMDA EPSCs. These results have lent support to the hypothesis that at some excitatory synapses diffusion, and not uptake, is primarily responsible for rapid dissipation of the glutamate within the synaptic cleft. The dependence on uptake is often increased when high intensity or high frequency stimuli are applied, or if the probability of release is artificially increased. Under such circumstances, prolongation of synaptic responses occurs at synapses in the hippocampus and the cerebellum when transporters are inhibited. These manipulations increase the probability that adjacent synapses release glutamate, and perhaps saturate transporters, resulting in enhanced spillout of transmitter from the synaptic cleft. The net effect is spillover of transmitter onto receptors at adjacent synapses, and pooling of transmitter between neighboring synapses (crosstalk). Similarly, receptors that are located further away from release sites, such as perisynaptic or presynaptic metabotropic receptors, are enhanced by transporter inhibition.
NMDA EPSCs. These results have lent support to the hypothesis that at some excitatory synapses diffusion, and not uptake, is primarily responsible for rapid dissipation of the glutamate within the synaptic cleft. The dependence on uptake is often increased when high intensity or high frequency stimuli are applied, or if the probability of release is artificially increased. Under such circumstances, prolongation of synaptic responses occurs at synapses in the hippocampus and the cerebellum when transporters are inhibited. These manipulations increase the probability that adjacent synapses release glutamate, and perhaps saturate transporters, resulting in enhanced spillout of transmitter from the synaptic cleft. The net effect is spillover of transmitter onto receptors at adjacent synapses, and pooling of transmitter between neighboring synapses (crosstalk). Similarly, receptors that are located further away from release sites, such as perisynaptic or presynaptic metabotropic receptors, are enhanced by transporter inhibition.
Of the transporters that have been identified, GLAST and GLT-1 are primarily expressed by astroglial cells (astrocytes and Bergmann glial cells), and have similar properties; measurements of Km in heterologous expression systems, or native membranes indicate that these transporters have an affinity for glutamate between 15 and 80 µM. Astroglial cells are optimized for glutamate uptake, due to their high resting potential and low cytoplasmic concentration of glutamate. The density of glutamate transporters in hippocampal astrocytes has been estimated to be between 2,500/µm2 and ~10,000/µm2. The high density of transporters in astrocyte membranes, the high capacity of astrocytes for glutamate uptake, and the close association of astrocyte processes with synapses, suggests that glial transporters are important for removing glutamate released at synapses. This hypothesis is supported by the activation of these transporters following stimulation of glutamatergic afferents, the dramatic loss in glutamate uptake capacity of brain tissue taken from animals treated with antisense against GLT-1, and the abnormal 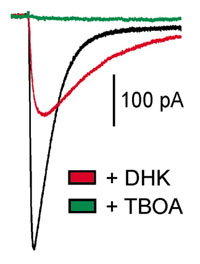 neurological symptoms of animals that have had the expression of GLT-1 transporters disrupted. In contrast, transgenic mice lacking EAAC1, the predominant neuronal glutamate transporter, have no CNS phenotype, and the seizure activity observed in adult animals treated with antisense to EAAC1 has been attributed to insufficient GABA synthesis due to a reduction in the uptake of glutamate into GABAergic terminals. These data support the hypothesis that astrocytes play an essential role in removing glutamate that is released during excitatory transmission.
neurological symptoms of animals that have had the expression of GLT-1 transporters disrupted. In contrast, transgenic mice lacking EAAC1, the predominant neuronal glutamate transporter, have no CNS phenotype, and the seizure activity observed in adult animals treated with antisense to EAAC1 has been attributed to insufficient GABA synthesis due to a reduction in the uptake of glutamate into GABAergic terminals. These data support the hypothesis that astrocytes play an essential role in removing glutamate that is released during excitatory transmission.
There are many unresolved questions about the role of glutamate transporters in synaptic signaling. Although four glutamate transporters are expressed in the brain, termed EAAT1-4 (EAAT5 is found only in the retina), we do not yet know what the individual contribution of these transporters is to glutamate clearance. In vitro experiments suggest that glutamate transporters may require tens of milliseconds to complete a cycle, though there have not yet been any measurements of transporter cycling in intact tissue under physiological conditions. In addition, critical questions remain to be answered regarding the efficiency of transporters (the probability that when a transporter binds a molecule of glutamate, that this molecule is translocated and released into the cytosol), and the speed with which glutamate is removed from the extracellular space.
Ongoing projects in the lab include studies of the biophysical properties of astroglial transporters (GLT-1 and GLAST), the pathways involved in regulating the activity and expression of glutamate transporters, and the role of specific transporters in excitatory synaptic transmission in the hippocampus, cerebellum, and in the cochlea of the mammalian ear. We are also developing new methods for monitoring transporter activity using fluorescent imaging. We rely primarily on whole-cell and patch voltage-clamp recording techniques, which are combined with infrared-DIC imaging to visualize cells in acute brain slices. Additional approaches involve the rapid application of solutions to outside-out patches using piezoelectric bimorphs, and imaging of intracellular Ca2+ with laser scanning confocal microscopy.