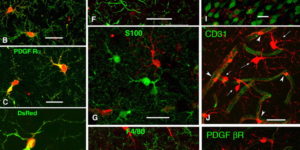
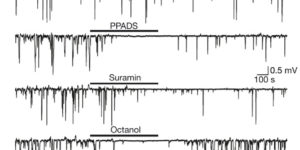
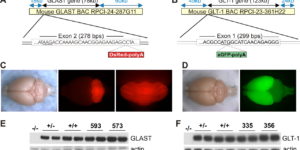
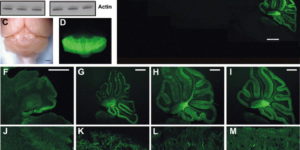
NG2 cells generate both oligodendrocytes and gray matter astrocytes
NG2 glia constitute a fourth major glial cell type in the mammalian central nervous system (CNS) that is distinct from other cell types. Although circumstantial evidence suggests that some NG2 glia differentiate into oligodendrocytes, their in vivo fate has not been directly examined. We have used the bacterial artificial chromosome (BAC) modification technique to generate transgenic mice that express DsRed or Cre specifically in NG2-expressing (NG2+) cells. In NG2DsRedBAC transgenic mice, DsRed was expressed specifically in NG2+ cells throughout the postnatal CNS. When the differentiation potential of NG2+ cells in vitro was examined using DsRed+NG2+ cells purified from perinatal transgenic brains, the majority of the cells either remained as NG2+ cells or differentiated into oligodendrocytes. In addition, DsRed+NG2+ cells also differentiated into astrocytes. The in vivo fate of NG2 glia was examined in mice that were double transgenic for NG2creBAC and the Cre reporter Z/EG. In the double transgenic mice, the Cre reporter EGFP was detected in myelinating oligodendrocytes and in a subpopulation of protoplasmic astrocytes in the gray matter of ventrolateral forebrain but not in fibrous astrocytes of white matter. These observations suggest that NG2+ cells are precursors of oligodendrocytes and some protoplasmic astrocytes in gray matter.