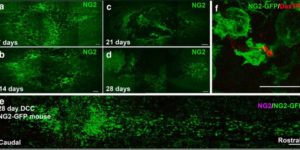
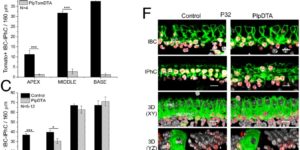
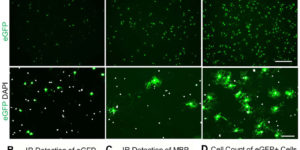
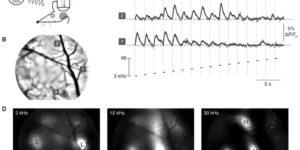
Large-scale recording of astrocyte activity.
Astrocytes are highly ramified glial cells found throughout the central nervous system (CNS). They express a variety of neurotransmitter receptors that can induce widespread chemical excitation, placing these cells in an optimal position to exert global effects on brain physiology. However, the activity patterns of only a small fraction of astrocytes have been examined and techniques to manipulate their behavior are limited. As a result, little is known about how astrocytes modulate CNS function on synaptic, microcircuit, or systems levels. Here, we review current and emerging approaches for visualizing and manipulating astrocyte activity in vivo. Deciphering how astrocyte network activity is controlled in different physiological and pathological contexts is crucial for defining their roles in the healthy and diseased CNS.