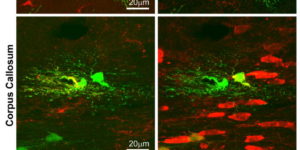
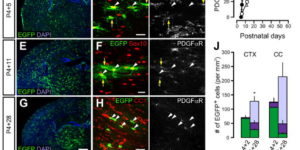
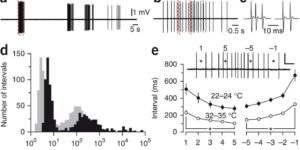
NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination.
Oligodendrocyte precursor cells (OPCs) express NMDA receptors (NMDARs) and form synapses with glutamatergic neurons throughout the CNS. Although glutamate influences the proliferation and maturation of these progenitors in vitro, the role of NMDAR signaling in oligodendrogenesis and myelination in vivo is not known. Here, we investigated the consequences of genetically deleting the obligatory NMDAR subunit NR1 from OPCs and their oligodendrocyte progeny in the CNS of developing and mature mice. NMDAR-deficient OPCs proliferated normally, achieved appropriate densities in gray and white matter, and differentiated to form major white matter tracts without delay. OPCs also retained their characteristic physiological and morphological properties in the absence of NMDAR signaling and were able to form synapses with glutamatergic axons. However, expression of calcium-permeable AMPA receptors (AMPARs) was enhanced in NMDAR-deficient OPCs. These results suggest that NMDAR signaling is not used to control OPC development but to regulate AMPAR-dependent signaling with surrounding axons, pointing to additional functions for these ubiquitous glial cells.