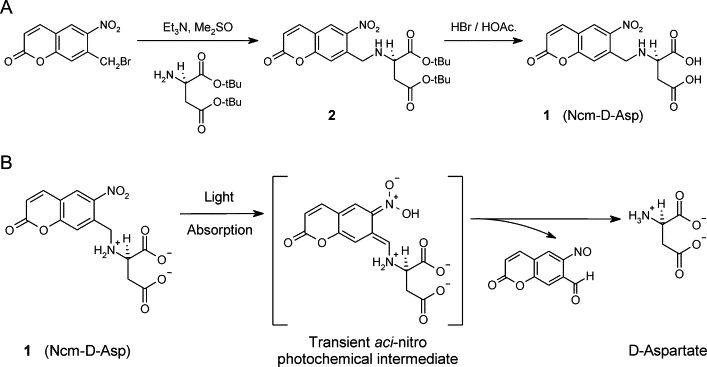
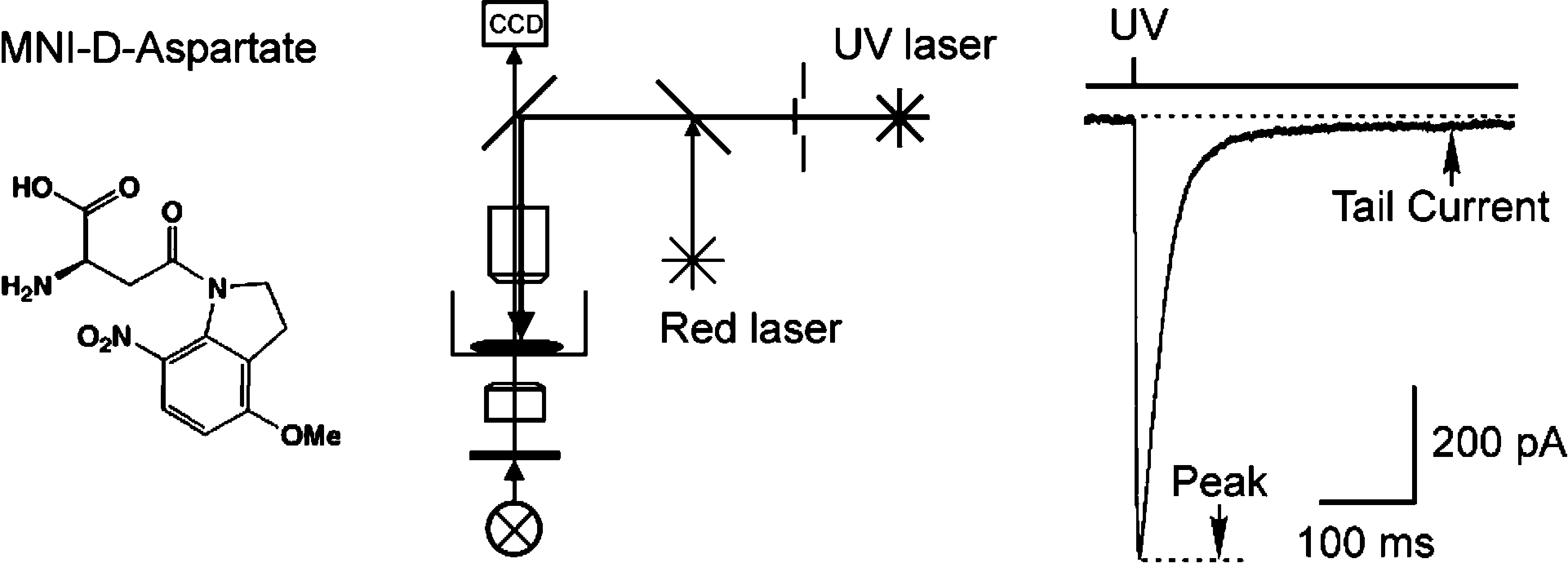
Ncm-D-aspartate: a novel caged D-aspartate suitable for activation of glutamate transporters and N-methyl-D-aspartate (NMDA) receptors in brain tissue
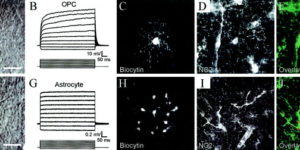
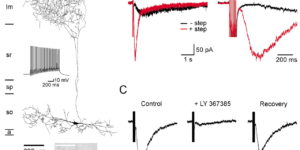
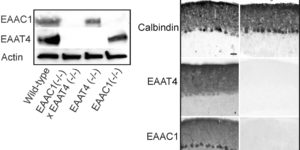
The D-isomer of aspartate is both a substrate for glutamate transporters and an agonist of N-methyl-D-aspartate (NMDA) receptors. To monitor the behavior of these receptors and transporters in intact tissue we synthesized a new photo-labile analogue of D-aspartate, N-[(6-nitrocoumarin-7-yl)methyl]-D-aspartic acid (Ncm-D-aspartate). This compound was photolyzed rapidly (t(1/2)=0.11 micros) by UV light with a quantum efficiency of 0.041 at pH 7.4. In acute hippocampal slices, photolysis of Ncm-D-aspartate by brief (1 ms) exposure to UV light elicited rapidly activating inward currents in astrocytes that were sensitive to inhibition by the glutamate transporter antagonist DL-threo-beta-benzyloxyaspartic acid (TBOA). Neither Ncm-D-aspartate nor the photo-released caging group exhibited agonist or antagonist activity at glutamate transporters, and Ncm-D-aspartate did not induce transporter currents prior to photolysis. Glutamate transporter currents were also elicited in cerebellar Purkinje cells in response to photolysis of Ncm-D-aspartate. Photo-release of D-aspartate from Ncm-D-aspartate did not induce alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainate receptor or metabotropic glutamate receptor (mGluR) currents, but triggered robust NMDA receptor currents in neurons; Ncm-D-aspartate and the photolzyed caging group were similarly inert at NMDA receptors. These results indicate that Ncm-D-aspartate can be used to study NMDA receptors at excitatory synapses and interactions between transporters and receptors in brain tissue.