Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain.
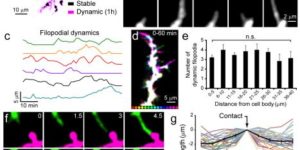
The adult CNS contains an abundant population of oligodendrocyte precursor cells (NG2(+) cells) that generate oligodendrocytes and repair myelin, but how these ubiquitous progenitors maintain their density is unknown. We generated NG2-mEGFP mice and used in vivo two-photon imaging to study their dynamics in the adult brain. Time-lapse imaging revealed that NG2(+) cells in the cortex were highly dynamic; they surveyed their local environment with motile filopodia, extended growth cones and continuously migrated. They maintained unique territories though self-avoidance, and NG2(+) cell loss though death, differentiation or ablation triggered rapid migration and proliferation of adjacent cells to restore their density. NG2(+) cells recruited to sites of focal CNS injury were similarly replaced by a proliferative burst surrounding the injury site. Thus, homeostatic control of NG2(+) cell density through a balance of active growth and self-repulsion ensures that these progenitors are available to replace oligodendrocytes and participate in tissue repair.