Zones of enhanced glutamate release from climbing fibers in the mammalian cerebellum.
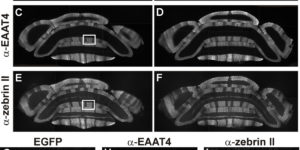
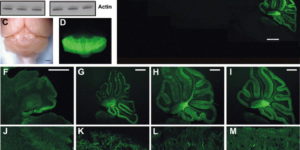
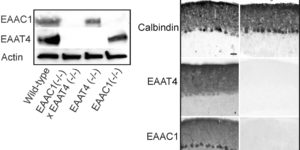
Purkinje cells in the mammalian cerebellum are remarkably homogeneous in shape and orientation, yet they exhibit regional differences in gene expression. Purkinje cells that express high levels of zebrin II (aldolase C) and the glutamate transporter EAAT4 cluster in parasagittal zones that receive input from distinct groups of climbing fibers (CFs); however, the physiological properties of CFs that target these molecularly distinct Purkinje cells have not been determined. Here we report that CFs that innervate Purkinje cells in zebrin II-immunoreactive (Z(+)) zones release more glutamate per action potential than CFs in Z(-) zones. CF terminals in Z(+) zones had larger pools of release-ready vesicles, exhibited enhanced multivesicular release, and produced larger synaptic glutamate transients. As a result, CF-mediated EPSCs in Purkinje cells decayed more slowly in Z(+) zones, which triggered longer-duration complex spikes containing a greater number of spikelets. The differences in the duration of CF EPSCs between Z(+) and Z(-) zones persisted in EAAT4 knock-out mice, indicating that EAAT4 is not required for maintaining this aspect of CF function. These results indicate that the organization of the cerebellum into discrete longitudinal zones is defined not only by molecular phenotype of Purkinje cells within zones, but also by the physiological properties of CFs that project to these distinct regions. The enhanced release of glutamate from CFs in Z(+) zones may alter the threshold for synaptic plasticity and prolong inhibition of cerebellar output neurons in deep cerebellar nuclei.