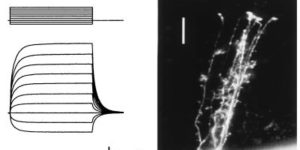
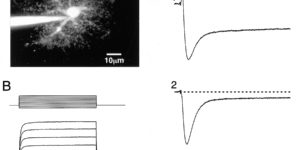
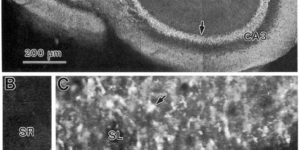
Glutamate transporter currents in Bergmann glial cells follow the time course of extrasynaptic glutamate.
Glutamate transporters in the central nervous system are expressed in both neurons and glia, they mediate high affinity, electrogenic uptake of glutamate, and they are associated with an anion conductance that is stoichiometrically uncoupled from glutamate flux. Although a complete cycle of transport may require 50-100 ms, previous studies suggest that transporters can alter synaptic currents on a much faster time scale. We find that application of L-glutamate to outside-out patches from cerebellar Bergmann glia activates anion-potentiated glutamate transporter currents that activate in <1 ms, suggesting an efficient mechanism for the capture of extrasynaptic glutamate. Stimulation in the granule cell layer in cerebellar slices elicits all or none alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor and glutamate transporter currents in Bergmann glia that have a rapid onset, suggesting that glutamate released from climbing fiber terminals escapes synaptic clefts and reaches glial membranes shortly after release. Comparison of the concentration dependence of both alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor and glutamate transporter kinetics in patches with the time course of climbing fiber-evoked responses indicates that the glutamate transient at Bergmann glial membranes reaches a lower concentration than attained in the synaptic cleft and remains elevated in the extrasynaptic space for many milliseconds.