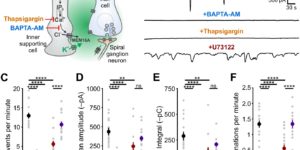
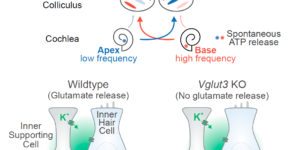
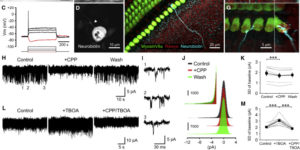
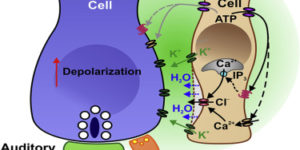
Purinergic Signaling in Cochlear Supporting Cells Reduces Hair Cell Excitability by Increasing the Extracellular Space
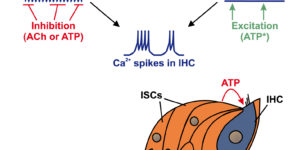
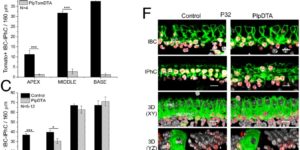
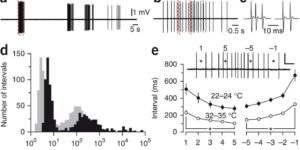
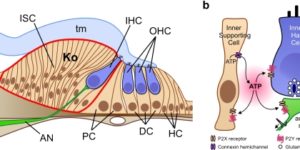
Neurons in developing sensory pathways exhibit spontaneous bursts of electrical activity that are critical for survival, maturation and circuit refinement. In the auditory system, intrinsically generated activity arises within the cochlea, but the molecular mechanisms that initiate this activity remain poorly understood. We show that burst firing of mouse inner hair cells prior to hearing onset requires P2RY1 autoreceptors expressed by inner supporting cells. P2RY1 activation triggers K+ efflux and depolarization of hair cells, as well as osmotic shrinkage of supporting cells that dramatically increased the extracellular space and speed of K+ redistribution. Pharmacological inhibition or genetic disruption of P2RY1 suppressed neuronal burst firing by reducing K+ release, but unexpectedly enhanced their tonic firing, as water resorption by supporting cells reduced the extracellular space, leading to K+ accumulation. These studies indicate that purinergic signaling in supporting cells regulates hair cell excitability by controlling the volume of the extracellular space.