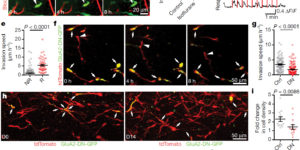
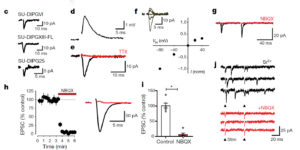
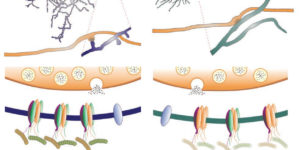
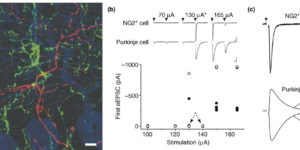
Glutamatergic synaptic input to glioma cells drives brain tumour progression.
A network of communicating tumour cells that is connected by tumour microtubes mediates the progression of incurable gliomas. Moreover, neuronal activity can foster malignant behaviour of glioma cells by non-synaptic paracrine and autocrine mechanisms. Here we report a direct communication channel between neurons and glioma cells in different disease models and human tumours: functional bona fide chemical synapses between presynaptic neurons and postsynaptic glioma cells. These neurogliomal synapses show a typical synaptic ultrastructure, are located on tumour microtubes, and produce postsynaptic currents that are mediated by glutamate receptors of the AMPA subtype. Neuronal activity including epileptic conditions generates synchronised calcium transients in tumour-microtube-connected glioma networks. Glioma-cell-specific genetic perturbation of AMPA receptors reduces calcium-related invasiveness of tumour-microtube-positive tumour cells and glioma growth. Invasion and growth are also reduced by anaesthesia and the AMPA receptor antagonist perampanel, respectively. These findings reveal a biologically relevant direct synaptic communication between neurons and glioma cells with potential clinical implications.