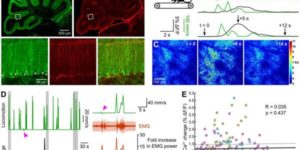
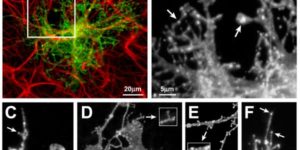
Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin.
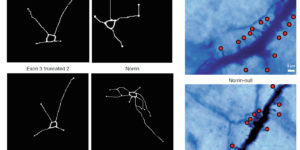
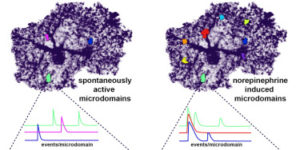
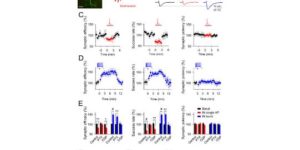
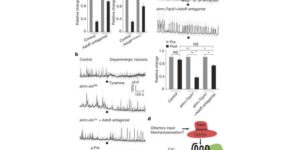
Despite expanding knowledge regarding the role of astroglia in regulating neuronal function, little is known about regional or functional subgroups of brain astroglia and how they may interact with neurons. We use an astroglia-specific promoter fragment in transgenic mice to identify an anatomically defined subset of adult gray matter astroglia. Using transcriptomic and histological analyses, we generate a combinatorial profile for the in vivo identification and characterization of this astroglia subpopulation. These astroglia are enriched in mouse cortical layer V; express distinct molecular markers, including Norrin and leucine-rich repeat-containing G-protein-coupled receptor 6 (LGR6), with corresponding layer-specific neuronal ligands; are found in the human cortex; and modulate neuronal activity. Astrocytic Norrin appears to regulate dendrites and spines; its loss, as occurring in Norrie disease, contributes to cortical dendritic spine loss. These studies provide evidence that human and rodent astroglia subtypes are regionally and functionally distinct, can regulate local neuronal dendrite and synaptic spine development, and contribute to disease.