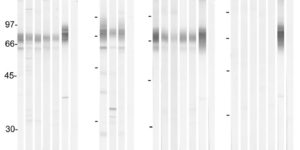
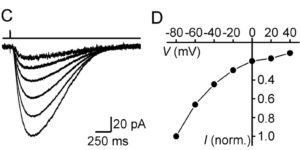
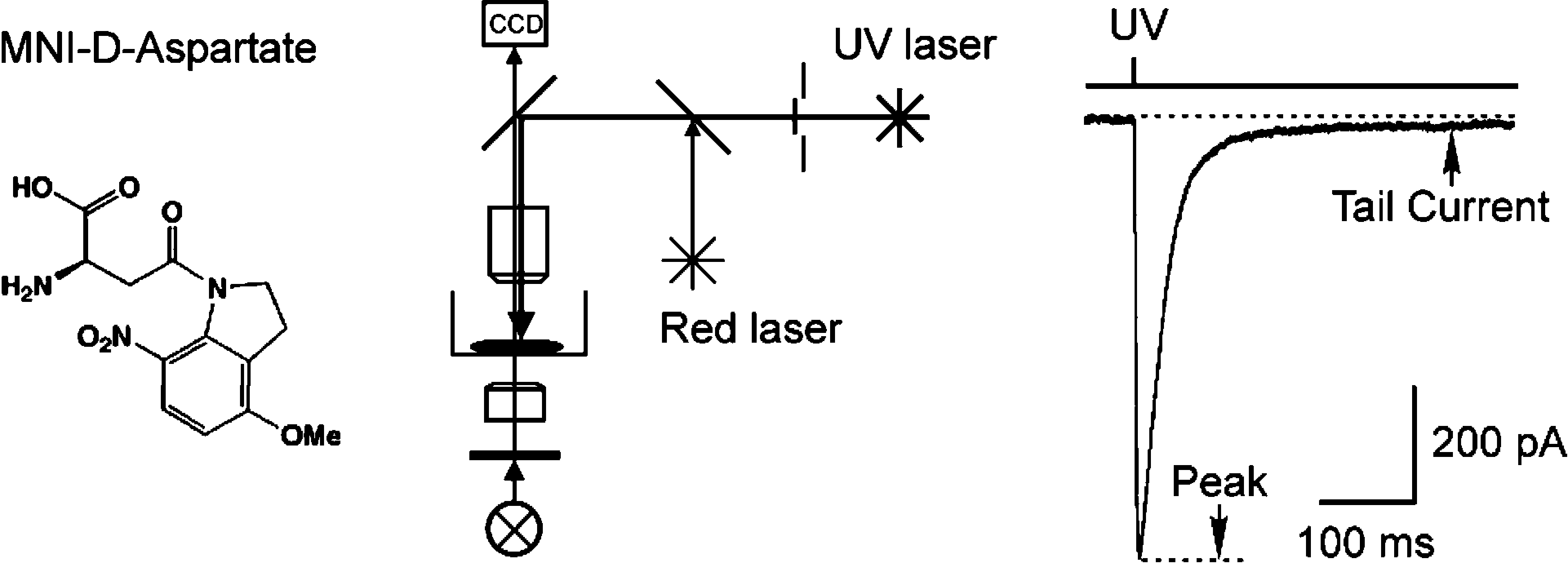
The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS.
The extracellular levels of excitatory amino acids are kept low by the action of the glutamate transporters. Glutamate/aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) are the most abundant subtypes and are essential for the functioning of the mammalian CNS, but the contribution of the EAAC1 subtype in the clearance of synaptic glutamate has remained controversial, because the density of this transporter in different tissues has not been determined. We used purified EAAC1 protein as a standard during immunoblotting to measure the concentration of EAAC1 in different CNS regions. The highest EAAC1 levels were found in the young adult rat hippocampus. Here, the concentration of EAAC1 was ∼0.013 mg/g tissue (∼130 molecules μm⁻³), 100 times lower than that of GLT-1. Unlike GLT-1 expression, which increases in parallel with circuit formation, only minor changes in the concentration of EAAC1 were observed from E18 to adulthood. In hippocampal slices, photolysis of MNI-D-aspartate (4-methoxy-7-nitroindolinyl-D-aspartate) failed to elicit EAAC1-mediated transporter currents in CA1 pyramidal neurons, and D-aspartate uptake was not detected electron microscopically in spines. Using EAAC1 knock-out mice as negative controls to establish antibody specificity, we show that these relatively small amounts of EAAC1 protein are widely distributed in somata and dendrites of all hippocampal neurons. These findings raise new questions about how so few transporters can influence the activation of NMDA receptors at excitatory synapses.