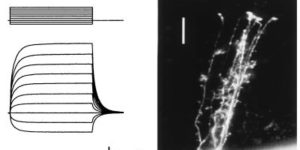
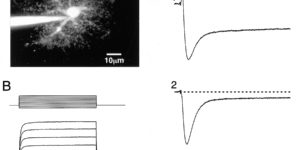
Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons.
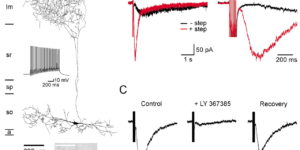
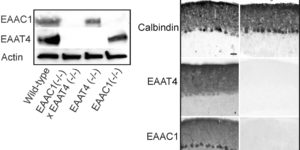
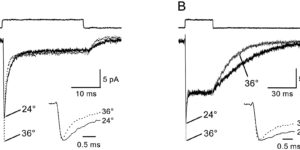
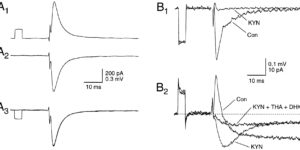
Clearance of extracellular glutamate is essential for limiting the activity of metabotropic glutamate receptors (mGluRs) at excitatory synapses; however, the relative contribution of transporters found in neuronal and glial membranes to this uptake is poorly understood. Hippocampalinterneurons located at the oriens-alveus border express mGluR1alpha, a metabotropic glutamate receptor that regulates excitability and synaptic plasticity. To determine which glutamate transporters are essential for removing glutamate at these excitatory synapses, we recorded mGluR1-mediated EPSCs from oriens-lacunosum moleculare (O-LM) interneurons in acute hippocampal slices. Stimulation in stratum oriens reliably elicited a slow mGluR1-mediated current in O-LM interneurons if they were briefly depolarized to allow Ca2+ entry before stimulation. Selective inhibition of GLT-1 [for glutamate transporter; EAAT2 (for excitatory amino acid transporter)] with dihydrokainate increased the amplitude of these responses approximately threefold, indicating that these transporters compete with mGluRs for synaptically released glutamate. However, inhibition of all glutamate transporters with TBOA (DL-threo-b-benzyloxyaspartic acid) increased mGluR1 EPSCs >15-fold, indicating that additional transporters also shape activation of these receptors. To identify these transporters, we examined mGluR1 EPSCs in mice lacking GLAST (for glutamate-aspartate transporter; EAAT1) or EAAC1 (for excitatory amino acid carrier; EAAT3). A comparison of responses recorded from wild-type and transporter knock-out mice revealed that the astroglial glutamate transporters GLT-1 and GLAST, but not the neuronal transporter EAAC1, restrict activation of mGluRs in O-LM interneurons. Transporter-dependent potentiation of mGluR1 EPSCs led to a dramatic increase in interneuron firing and enhanced inhibition of CA1 pyramidal neurons, suggesting that acute or prolonged disruption of transporter activity could lead to changes in network activity as a result of enhanced interneuron excitability.