Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility
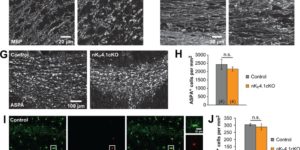
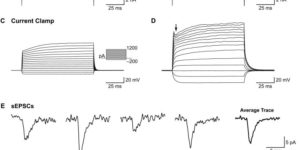
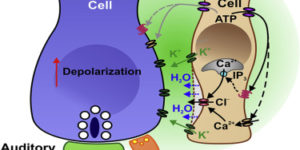
The inwardly rectifying K+ channel Kir4.1 is broadly expressed by CNS glia and deficits in Kir4.1 lead to seizures and myelin vacuolization. However, the role of oligodendrocyte Kir4.1 channels in controlling myelination and K+ clearance in white matter has not been defined. Here, we show that selective deletion of Kir4.1 from oligodendrocyte progenitors (OPCs) or mature oligodendrocytes did not impair their development or disrupt the structure of myelin. However, mice lacking oligodendrocyte Kir4.1 channels exhibited profound functional impairments, including slower clearance of extracellular K+ and delayed recovery of axons from repetitive stimulation in white matter, as well as spontaneous seizures, a lower seizure threshold, and activity-dependent motor deficits. These results indicate that Kir4.1 channels in oligodendrocytes play an important role in extracellular K+ homeostasis in white matter, and that selective loss of this channel from oligodendrocytes is sufficient to impair K+ clearance and promote seizures.