Homeostatic Control of Spontaneous Activity in the Developing Auditory System.
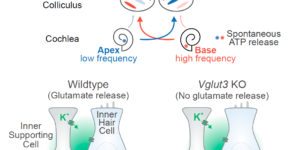
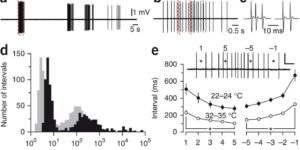
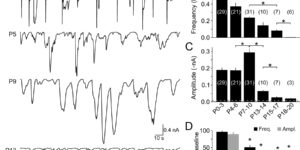
Neurons in the developing auditory system exhibit spontaneous bursts of activity before hearing onset. How this intrinsically generated activity influences development remains uncertain, because few mechanistic studies have been performed in vivo. We show using macroscopic calcium imaging in unanesthetized mice that neurons responsible for processing similar frequencies of sound exhibit highly synchronized activity throughout the auditory system during this critical phase of development. Spontaneous activity normally requires synaptic excitation of spiral ganglion neurons (SGNs). Unexpectedly, tonotopic spontaneous activity was preserved in a mouse model of deafness in which glutamate release from hair cells is abolished. SGNs in these mice exhibited enhanced excitability, enabling direct neuronal excitation by supporting cell-induced potassium transients. These results indicate that homeostatic mechanisms maintain spontaneous activity in the pre-hearing period, with significant implications for both circuit development and therapeutic approaches aimed at treating congenital forms of deafness arising through mutations in key sensory transduction components.