Changes in the Oligodendrocyte Progenitor Cell Proteome With Ageing
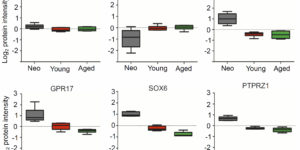
Following central nervous system (CNS) demyelination, adult oligodendrocyte progenitor cells (OPCs) can differentiate into new myelin-forming oligodendrocytes in a regenerative process called remyelination. While remyelination is very efficient in young adults, its efficiency declines progressively with ageing. Here we performed proteomic analysis of OPCs freshly isolated from the brains of neonate, young and aged female rats. Approximately 50% of the proteins are expressed at different levels in OPCs from neonates compared to their adult counterparts. The amount of myelin-associated proteins, and proteins associated with oxidative phosphorylation, inflammatory responses and actin cytoskeletal organization increased with age, while cholesterol-biosynthesis, transcription factors and cell cycle proteins decreased. Our experiments provide the first ageing OPC proteome, revealing the distinct features of OPCs at different ages. These studies provide new insights into why remyelination efficiency declines with ageing and potential roles for aged OPCs in other neurodegenerative diseases.