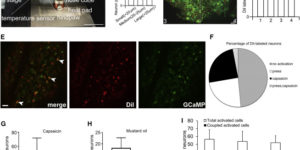
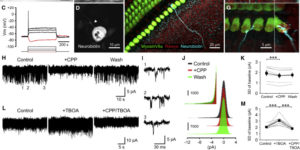
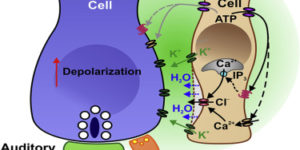
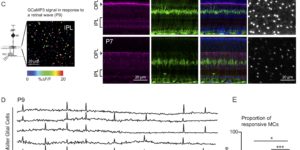
Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain.
Primary sensory neurons in the DRG play an essential role in initiating pain by detecting painful stimuli in the periphery. Tissue injury can sensitize DRG neurons, causing heightened pain sensitivity, often leading to chronic pain. Despite the functional importance, how DRG neurons function at a population level is unclear due to the lack of suitable tools. Here we developed an imaging technique that allowed us to simultaneously monitor the activities of >1,600 neurons/DRG in live mice and discovered a striking neuronal coupling phenomenon that adjacent neurons tend to activate together following tissue injury. This coupled activation occurs among various neurons and is mediated by an injury-induced upregulation of gap junctions in glial cells surrounding DRG neurons. Blocking gap junctions attenuated neuronal coupling and mechanical hyperalgesia. Therefore, neuronal coupling represents a new form of neuronal plasticity in the DRG and contributes to pain hypersensitivity by “hijacking” neighboring neurons through gap junctions.