Synergistic Signaling by Light and Acetylcholine in Mouse Iris Sphincter Muscle
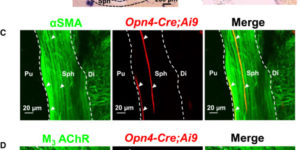
The mammalian pupillary light reflex (PLR) involves a bilateral brain circuit whereby afferent light signals in the optic nerve ultimately drive iris-sphincter-muscle contraction via excitatory cholinergic parasympathetic innervation. Additionally, the PLR in nocturnal and crepuscular sub-primate mammals has a “local” component in the isolated sphincter muscle, as in amphibians, fish, and bird. In mouse, this local PLR requires the pigment melanopsin, originally found in intrinsically photosensitive retinal ganglion cells (ipRGCs). However, melanopsin’s presence and effector pathway locally in the iris remain uncertain. The sphincter muscle itself may express melanopsin, or its cholinergic parasympathetic innervation may be modulated by suggested intraocular axonal collaterals of ipRGCs traveling to the eye’s ciliary body or even to the iris. Here, we show that the muscarinic receptor antagonist, atropine, eliminated the effect of acetylcholine (ACh), but not of light, on isolated mouse sphincter muscle. Conversely, selective genetic deletion of melanopsin in smooth muscle mostly removed the light-induced, but not the ACh-triggered, increase in isolated sphincter muscle’s tension and largely suppressed the local PLR in vivo. Thus, sphincter muscle cells are bona fide, albeit unconventional, photoreceptors. We found melanopsin expression in a small subset of mouse iris sphincter muscle cells, with the light-induced contractile signal apparently spreading through gap junctions into neighboring muscle cells. Light and ACh share a common signaling pathway in sphincter muscle. In summary, our experiments have provided details of a photosignaling process in the eye occurring entirely outside the retina.