Physiological characteristics of NG2-expressing glial cells.
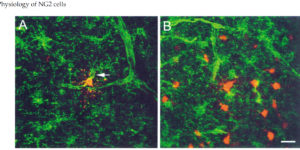
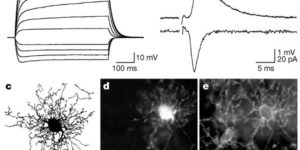
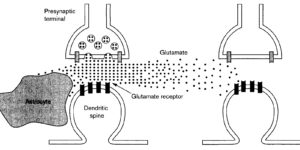
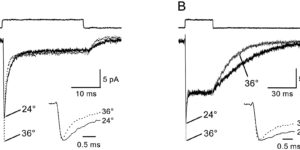
Antibodies against the chondroitin sulfate proteoglycan NG2 label a subpopulation of glial cells within the CNS, which have a small cell body and thin radiating processes. Physiological recordings from these small cells in acute brain slices have revealed that they possess unique properties, suggesting that they may comprise a class of glial cells distinct from astrocytes, oligodendrocytes, or microglia. NG2-expressing glial cells (abbreviated as “NG2 cells” here) have a moderate input resistance and are not dye- or tracer-coupled to adjacent cells. They express voltage-gated Na+, K+ and Ca2+ conductances, though they do not exhibit regenerative Na+ or Ca2+ action potentials due to the much larger K+ conductances present. In addition to voltage-gated conductances, they express receptors for various neurotransmitters. In the hippocampus, AMPA and GABAA receptors on these cells are activated by release of transmitter from neurons at defined synaptic junctions that are formed with CA3 pyramidal neurons and GABAergic interneurons. These rapid forms of neuron-glial communication may regulate the proliferation rate of NG2 cells or their development into mature oligodendrocytes. These depolarizing inputs may also trigger the release of neuroactive substances from NG2 cells, providing feedback regulation of signaling at neuronal synapses. Although the presence of Ca2+ permeable AMPA receptors provides a pathway to link neuronal activity to Ca2+ dependent processes within the NG2 cells, these receptors also put these cells at risk for glutamate-associated excitotoxicity. This vulnerability to the sustained elevation of glutamate may underlie ischemic induced damage to white matter tracts and contribute to cerebral palsy in premature infants.