Specificity controls for immunocytochemistry: the antigen preadsorption test can lead to inaccurate assessment of antibody specificity.
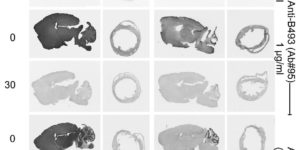
The biomedical research community relies directly or indirectly on immunocytochemical data. Unfortunately, validation of labeling specificity is difficult. A common specificity test is the preadsorption test. This test was intended for testing crude antisera but is now frequently used to validate monoclonal and affinity purified polyclonal antibodies. Here, the authors assess the power of this test. Nine affinity purified antibodies to different epitopes on 3 proteins (EAAT3, slc1a1; EAAT2, slc1a2; BGT1, slc6a12) were tested on samples (tissue sections and Western blots with or without fixation). The selected antibodies displayed some degree of cross-reactivity as defined by labeling of samples from knockout mice. The authors show that antigen preadsorption blocked all labeling of both wild-type and knockout samples, implying that preadsorption also blocked binding to cross-reactive epitopes. They show how this can give an illusion of specificity and illustrate sensitivity-specificity relationships, the importance of good negative controls, that fixation can create new epitopes, and that cross-reacting epitopes present in sections may not be present on Western blots and vice versa. In conclusion, they argue against uncritical use of the preadsorption test and, in doing so, address a number of other issues related to immunocytochemistry specificity testing.