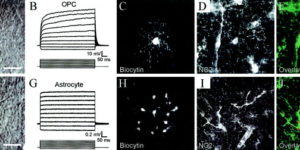
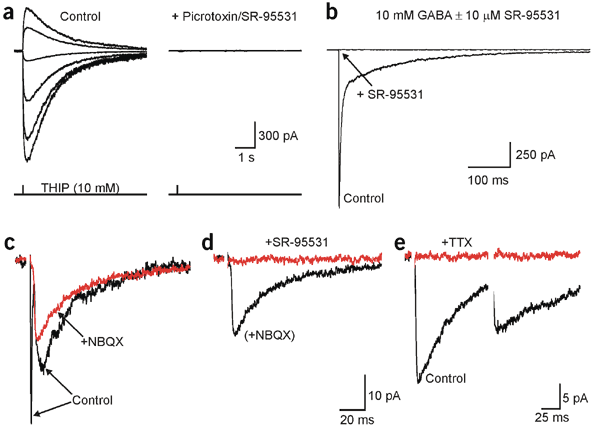
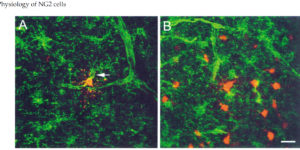
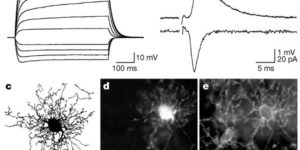
Synaptic signaling between neurons and glia.
Rapid signaling between vertebrate neurons occurs primarily at synapses, intercellular junctions where quantal release of neurotransmitter triggers rapid changes in membrane conductance through activation of ionotropic receptors. Glial cells express many of these same ionotropic receptors, yet little is known about how receptors in glial cells become activated in situ. Because synapses were thought to be the sole provenance of neurons, it has been assumed that these receptors must be activated following diffusion of transmitter out of the synaptic cleft, or through nonsynaptic mechanisms such as transporter reversal. Two recent reports show that a ubiquitous class of progenitors that express the proteoglycan NG2 (NG2 cells) engage in rapid signaling with glutamatergic and gamma-aminobutyric acid (GABA)ergic neurons through direct neuron-glia synapses. Quantal release of transmitter from neurons at these sites triggers rapid activation of aminomethylisoxazole propionic acid (AMPA) or GABA(A) receptors in NG2 cells. These currents exhibit properties consistent with direct rather than spillover-mediated transmission, and electron micrographic analyses indicate that nerve terminals containing clusters of synaptic vesicles form discrete junctions with NG2 cell processes. Although activation of AMPA or GABA(A) receptors depolarize NG2 cells, these receptors are more likely to serve as routes for ion flux rather than as current sources for depolarization, because the amplitudes of the synaptic transients are small and the resting membrane potential of NG2 cells is highly negative. The ability of both glutamate and GABA to influence the morphology, physiology, and development of NG2 cells in vitro suggests that this rapid form of signaling may play important roles in adapting the behavior of these cells to the needs of surrounding neurons in vivo.